
There’s also “less risk from contamination with live infectious reagents and the release of dangerous pathogens,” the research said. It also eliminates the dangers associated with the growing amount of dangerous pathogens and the need to produce vaccines at the scale needed to supply large populations. The study also found that manufacturing the new type of vaccine is safe and timesaving. On the other hand, vaccination with “non-viral delivered nucleic acid-based vaccines mimics infection or immunization with live microorganisms,” while stimulating a potent antibody immune response, according to the study. Without more information, the risks and benefits of taking the Chinese vaccine remain unclear. These include “the potential to cause disease in immuno-compromised individuals and the possibility of reversion to a virulent form,” the study authors said.
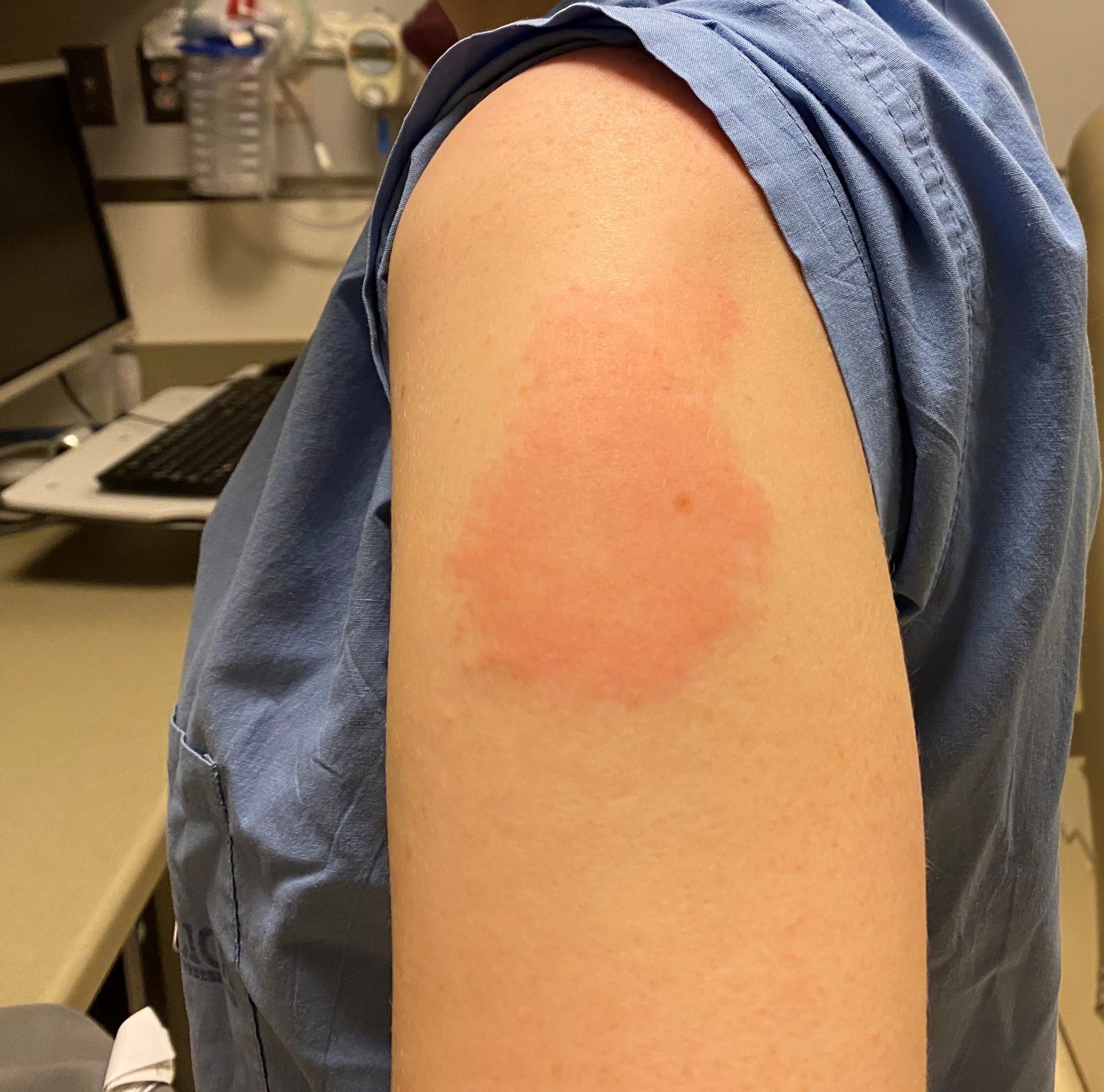
#CHINESE COVID VACCINE SIDE EFFECT TRIAL#
“The trial of the first mRNA product, which was not a human trial, was back in 1990, and showed some good efficacy.”Įlder said that in the early ’90s, a group of researchers used the same technology for a specific purpose and were able to demonstrate that “this technology was efficacious for the purposes that it was designed for.”Īccording to a 2019 study, while vaccines that use a weakened (or dead) version of the virus have provided enormous benefits in saving lives, this technology had significant drawbacks in the past. “This technology has actually been around since 1990,” he said. James Elder, internist at Texas Health Harris Methodist Hospital in Southwest Fort Worth, this new technology is actually decades old. “Other vaccines use a ‘crippled’ form of a whole virus to stimulate immunity like MMR, flu. Len Horovitz, a pulmonary specialist at Lenox Hill Hospital in New York, told Healthline. “mRNA is a portion of virus genetic information,” Dr.
Pfizer’s and Moderna’s vaccines use this technology.

The data “shows Beijing Institute of Biological Product’s inactivated vaccine to have 86 percent efficacy against COVID-19 infection,” MOHAP said. However, MOHAP and the Department of Health Abu Dhabi have reviewed an interim analysis of Sinopharm’s phase 3 trials, the ministry said in a statement. Sinopharm and the UAE have yet to release detailed data on a phase 3 trial of 31,000 participants to be widely verified by independent experts. 9.Īccording to the official press release, this vaccine has been granted emergency use authorization in the United Arab Emirates (UAE) since September to “protect frontline workers most at risk of COVID-19.” The United Arab Emirates approved the Chinese drugmaker Sinopharm’s COVID-19 vaccine candidate, the country’s Ministry of Health and Prevention (MOHAP) announced Dec.


 0 kommentar(er)
0 kommentar(er)
